3 new positive progress in cholesterol-lowering drugs
March 08, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Today, Esperion announced positive results for the first key phase 3 study of bempedoic acid. This study evaluated the efficacy, safety, and resistance of low-density lipoprotein cholesterol (LDL-C) in 180 mg bempedoic acid compared with placebo in patients with atherosclerotic cardiovascular disease (ASCVD) or high-risk ASCVD. Receptive.

High levels of LDL-C can lead to accumulation of fat and cholesterol in and around the arterial wall, known as atherosclerosis, which can lead to cardiovascular events including heart disease or stroke. In the United States, there are 78 million people or more than 20% of the population with higher LDL-C. There are 73 million people in Europe, 30 million people in Japan, and elevated LDL-C. There are approximately 13 million ASCVD patients in the United States, and even with the highest tolerated dose of lipid modification therapy (including those who are intolerant to statins), there is still a higher level of LDL-C, leading to a higher risk of cardiovascular events. The vast majority of these patients (9.5 million) need to reduce LDL-C by nearly 30% to achieve treatment goals.
Esperion is committed to providing patients with daily oral therapy to supplement existing treatments to help these patients achieve additional LDL-C goals. Bempedoic acid is a first-in-class, once daily, supplemental, oral ATP citrate lyase (ACL) inhibitor that lowers cholesterol biosynthesis and lowers LDL-C levels by upregulating LDL receptors. Similar to statins, bempedoic acid also reduces high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation associated with cardiovascular disease. Phase 1 and Phase 2 studies on bempedoic acid have been completed, monotherapy has reduced LDL-C by up to 30%, and combined with ezetimibe reduces LDL-C by about 50%, and can be added to stable statin therapy. Reduce it by 20%.
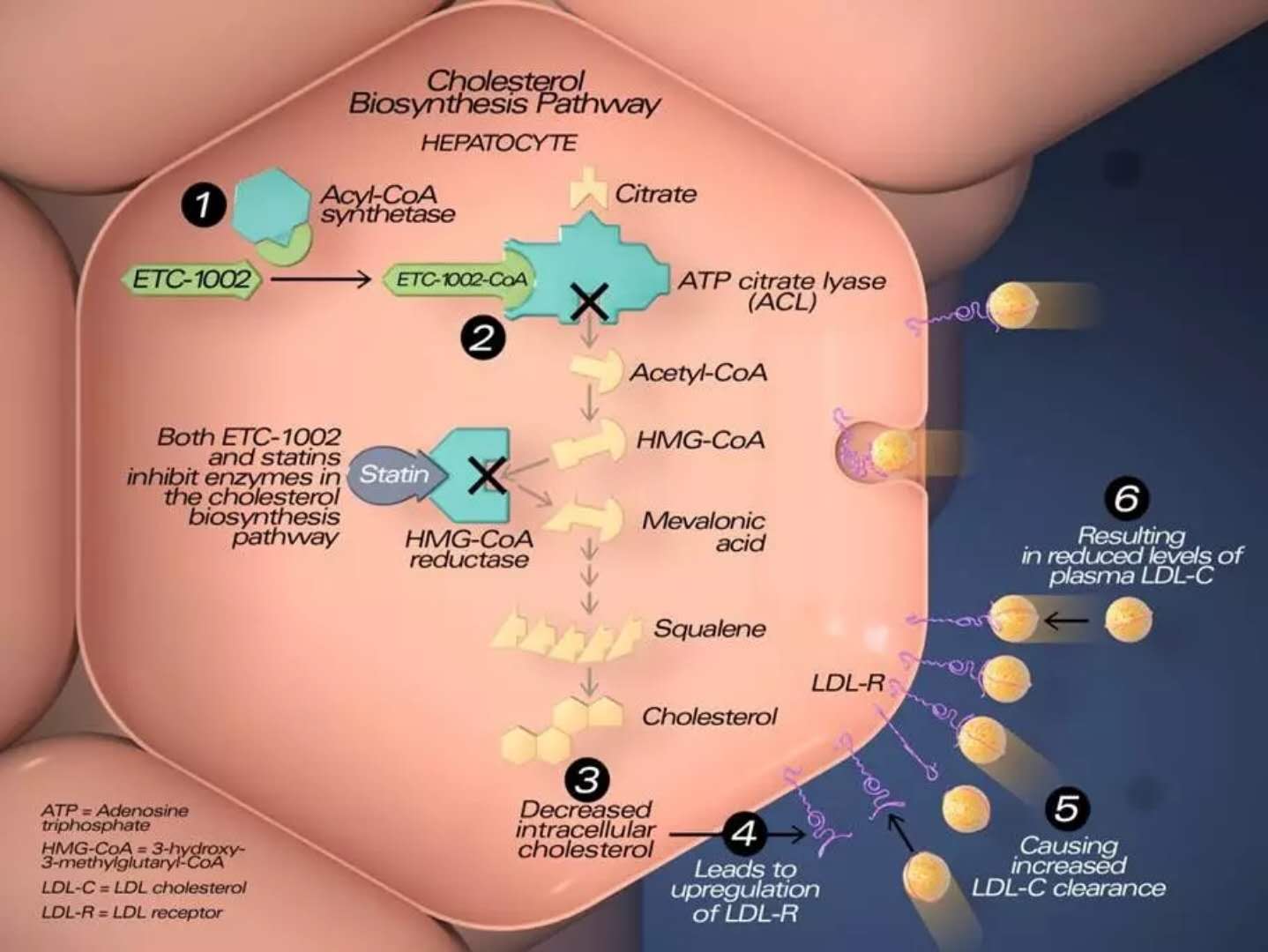
â–²The mechanism of action of Bempedoic acid (Source: Esperion official website)
To further validate the efficacy and safety of bempedoic acid, Esperion initiated a 12-week, randomized, double-blind, placebo-controlled, multicenter, global key phase 3 study enrolling 269 ASCVD patients or ASCVD with hypercholesterolemia In high-risk populations, these patients, although treated with the highest tolerated doses of statins and ezetimibe, still have higher LDL-C levels, leading to a higher risk of cardiovascular events. These patients were randomized to receive 180 mg of bempedoic acid or placebo in a 2:1 ratio. The primary endpoint was to reduce the efficacy of lep-C at the 12-week treatment with bempedoic acid. The secondary endpoint was to assess the safety and tolerability of bempedoic acid and its impact on other risk markers, including hsCRP.
The results showed that the study reached the primary endpoint and LDL-C decreased by 28% (p < 0.001). LDL-C was reduced by 23% from baseline in the Bempedoic acid group and by 5% in the placebo group. The hsCRP was also significantly reduced by 33% in the Bempedoic acid group and by 2% in the placebo group (p < 0.001). In addition, this study observed that bempedoic acid has better safety and tolerability.
Based on these results, Esperion plans to submit a new drug application (NDA) of the combination of bepopedoic acid and bempedoic acid/ezetimibe to the US FDA in the first quarter of 2019 to reduce LDL-C. In addition, Esperion plans to submit a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) in the second quarter of 2019.
“Many of my patients, including those who are considered to be intolerant of statins, are at risk for cardiovascular disease, including heart attacks and strokes, although there are available LDL-C treatments,†Esperion's Phase 3 Executive Committee Dr. Christie M. Ballantyne, Chair and Professor of Cardiology and Director of Baylor College of Medicine, said: "These results suggest that bepopedoic acid with a targeted mechanism and convenient daily oral administration may be an important new treatment option for a wide range of patients. Includes those who cannot tolerate moderate or high doses of commonly used statins."

â–² Mr. Tim M. Mayleben, President and CEO of Esperion (Source: Esperion Official Website)
“Doctors are eagerly awaiting a new daily oral therapy to supplement existing oral medications to reduce LDL-C in high-risk patients, with the convenience and tolerance that patients hope and deserve,†said Esperion President Mr. Tim M. Mayleben, CEO and CEO: “Looking ahead, we look forward to the results of the subsequent phase 3 clinical studies to further confirm that the combination of bempedoic acid and bempedoic acid/ezetimibe can pass safe, well tolerated and easy to take daily. A single dose that provides consistent LDL-C reduces efficacy and is highly complementary to existing standard treatment oral LDL-C reduction therapy."
Reference materials:
[1] Esperion Announces Positive Top-Line Results from First Pivotal Phase 3 Study of Bempedoic Acid
[2] Esperion's Cholesterol Drug Positive in PhIII Trial
Anesthesia and Breath Disposables
Central Venous Catheter,Hemodialysis Catheter,Blood Pressure Transducer,Phripheral Inerted Central Catheter,Artery Compression Device Sampler,Infusion Pump
Anesthesia Medical Co., Ltd. , https://www.jssinoanesthesias.com