
On May 18, 2016, BonaFire Cardiac Pacing Electrode was first used in the first-in-man (FIM) clinical trials of the heart rhythm management medical device (Shanghai) Co., Ltd. (hereinafter referred to as “Chuangling Heart Rhythm Medicalâ€). The implantation was successfully completed at Zhongshan Hospital affiliated to Fudan University. The patient's vital signs were stable after surgery, which means that the heart rhythm medical therapy has taken a solid step on the road of “innovation pacing and dreaming of Chinaâ€.
Medical workers combined to launch "Shanghai Creation", industry experts pay attention to expectations
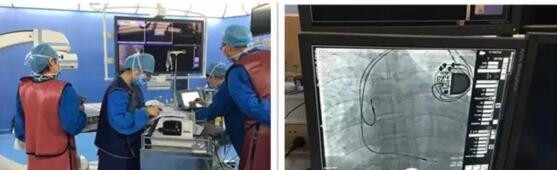
The BonaFire cardiac pacing electrode wire was independently developed and produced by Chuangxin Heart Rhythm Medical. During the period, it was strongly supported by the Shanghai Science and Technology Commission R&D Fund. The Zhongshan Hospital affiliated to Fudan University and Renji Hospital affiliated to Shanghai Jiaotong University participated in the research and development. After the operation, Professor Su Yangang from Zhongshan Hospital and Qin Shengmei, the surgeon of the implant, said: "BonaFire's pacing parameters and performance are consistent with the current imported products, and some of the structural design is even better than imported products. We are confident in its long-term stability and safety.†Professor Mao Jialiang of Renji Hospital repeatedly said in the early animal experiments: “BonaFire has no difference in implant operation and imported products. I believe that after its official launch, Most doctors can be proficient in application." Professor Ge Junbo, academician of the Chinese Academy of Sciences, director of the Department of Cardiology of Zhongshan Hospital, and Professor Ben Ben of the Department of Cardiology of Renji Hospital believe that: "This is another case in which the first-line clinical participation in medical device research and development has been successful. It fully proves that 'from clinical, clinical use' is the correct path that domestic high-end medical device research and development should follow.†The leaders of the Chinese Medical Association Electrocardiogram and Pacing Branch and the Chinese Medical Association's Heart Rhythm Committee are on different occasions. Expressed concern, support and expectation for domestic cardiac pacing products .
Poly-Lok Endo Appliers,Laparoscopic Hemoclip Applier,Vascular Clip Applicator,Surgical Clip Applier For Slae
Qingdao DMD Medical Technology Co., LTD , https://www.conston-tech.com