Release date: 2017-12-15

1. Developed countries and pharmaceutical giants increase investment in research and development of gene therapy
US funding for gene therapy continues to increase. In June 2014, the National Institutes of Health (NIH) funded $25 million for gene therapy research on infectious diseases (malaria and influenza). At the end of 2015, the White House released the "New Strategy for American Innovation", which clearly defines precision medicine, including gene therapy, as its future development strategy. It will invest 4.8 billion US dollars in key funding in the next 10 years.
European funding for gene therapy continues to increase. The EU research framework has established a special gene therapy funding program, the CliniGene (2006-2011) program, to contribute 65.8 million euros to promote the development of clinical gene therapy in Europe. Horizon 2020 is the EU's largest research and innovation framework program, with gene therapy receiving €49.1 million in funding. In June 2016, the French government announced an investment of 670 million euros to start genomic and individualized medical projects. The project will last for 10 years and will focus on genomics, individualized medicine, gene therapy and other research.
In addition, multinational pharmaceutical giants have invested heavily in gene therapy. In December 2014, Pfizer signed a cooperation agreement with Spark Therapeutics, a rare disease gene therapy company in the United States, to jointly develop gene therapy products for hemophilia B. In April 2015, Bristol-Myers Squibb (BMS) signed a $1 billion cooperation agreement with uniQure, a Dutch biotechnology company leader in gene therapy, to develop a recombinant adeno-associated virus (AAV) expression vector. Cardiovascular gene therapy drugs. In 2016, Baijian announced an investment of US$2 billion to develop a gene therapy drug based on AAV vectors in cooperation with the University of Pennsylvania and REGENEXBIO.
2. Gene therapy research and development and industrialization have made important progress
As of April 2017, 2463 gene therapy clinical trials were registered on the Clinical Trial website (Figure 2), including nearly 500 gene therapy clinical trials in Phase II/III, and a total of 7 gene therapy products have been Listed in the United States, the European Union, China and other countries. In July 2012, the European Medicines Agency (EMA) approved the launch of the AAV-based gene therapy drug Glybera, developed by uniQure in the European Union, for severe or repeated pancreatitis episodes that are severely restricted to high-fat diets. Treatment of patients with lipoprotein lipase deficiency (LPLD). In addition, in 2014-2015, Calledon, Spark Therapeutics and Bluebird Bio's products MYDICAR, SPK-RPE65 and LentiGlobin were approved for FDA listing. In May 2016, EMA approved GlaxoSmithKline (GSK) drug Strimvelis for the treatment of rare adenosine deaminase deficiency caused by severe combined immunodeficiency (ADA-SCID), the first in vitro gene to be marketed. Modified hematopoietic stem cell gene therapy products.
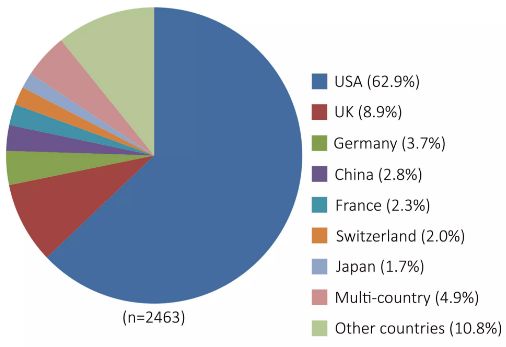
â–² Figure 2: Distribution statistics of clinical trials of gene therapy in major countries around the world
From August to November 2017, there were four milestones in the field of gene therapy. In August, the FDA was the first to approve Novartis's CAR-T cell therapy product, Kymriah, for the treatment of relapsed or refractory acute lymphoblastic leukemia. In October, the FDA's panel of experts approved the approval of Spark's AAV-based gene therapy drug AAV-RPE65 (Luxturna) for the treatment of retinal diseases caused by RPE65 gene defects, which can prevent patients from developing completely blind. In the same month, the FDA approved Kite Pharma's CAR-T gene therapy product, Yescarta, for the treatment of adult relapsed/refractory large B-cell lymphoma; November, Sangamo Therapeutics, USA The use of zinc finger nuclease (ZFN) technology to achieve the editing of the genome of the first patient in human history.
3. The main development trend of gene therapy research and development
(1) Recombinant adeno-associated virus (AAV) vector gene therapy. Recombinant AAV is derived from non-pathogenic wild-type adeno-associated virus. It has the characteristics of good safety, wide range of host cells, low immunogenicity, and long-term expression of foreign genes in vivo. It is the most promising gene therapy vector. one. In November 2017, the New England Journal of Medicine published a clinical trial: Recombinant AAV gene therapy successfully extended the lives of 15 children with severe hereditary disease type-1 spinal muscular atrophy (SMA1). Give them a chance to regain their health. In addition, there are a variety of AAV-based gene therapy drugs are being carried out in phase II or phase III clinical trials, almost all of which are monogenic hereditary rare diseases, including hemophilia A and B, thalassemia, Spinal muscular atrophy (SMA) and the like.
(2) Lentiviral vector (LV) gene therapy. LV is mainly used for in vitro transgenic research. LV has the advantages of being able to infect non-dividing cells, transferring a large amount of gene fragments, long expression time of the target gene, and not easily inducing host immune response, and is a viral vector with a relatively good application prospect. The vectors used for gene therapy drugs such as Strimvelis, Kymriah and Yescarta are all LV.
(3) Gene-modified oncolytic virus gene therapy. The development of malignant tumors such as oncolytic herpes virus, oncolytic adenovirus, and Newcastle disease virus carrying therapeutic genes is promising. In recent years, multinational pharmaceutical companies have laid out oncolytic virus projects. Multinational pharmaceutical companies such as Amgen, Pfizer and AstraZeneca are involved in the oncolytic virus project. For example, in 2011, Amgen acquired BioVex, a professional gene therapy company, for $1 billion (the main product is GM-CSF-modified herpesvirus OncoVEXGMCSF); in October 2015, the FDA approved the market for the treatment of melanoma. .
(4) Gene editing techniques such as CRISPR/Cas9 are used for gene therapy. In recent years, gene editing technology has developed rapidly and is revolutionizing the entire field of biotechnology. Compared with traditional gene therapy methods, gene editing technology can modify DNA sequences at the genomic level to repair genetic defects or change cell functions, making it possible to completely cure malignant diseases such as leukemia, AIDS and hemophilia. Two anti-AIDS and one type B hemophilia products based on zinc finger nuclease (ZFN) gene editing technology have entered the clinical stage. The CRISPR/Cas9 gene editing technology is more accurate and less expensive for specific genomic DNA and is becoming the mainstream technology for basic research and clinical applications (Figure 3). Recently, the United States, the United Kingdom, and the Japanese government have liberalized the restriction on genetically modified human embryos, and carried out a series of genetic editing techniques to modify embryos to conduct gene therapy research on congenital genetic diseases.
â–² Figure 3 - Modifying genomic DNA using genetic scissors (CRISPR)
4. Status of domestic science and technology development
The Chinese government attaches great importance to the research of basic research, target products and key technologies related to gene therapy. During the “Eleventh Five-Year Plan†and “Twelfth Five-Year Plan†period, the 863 Program has specially set up special projects for gene therapy of major diseases. A number of domestic advantageous units engaged in biotherapeutic research, more than 10 national key laboratories and a number of professional companies have stockpiled a number of bio-therapeutic technologies and projects with independent intellectual property rights. At the same time, the 973 program also funded the basic research and applied basic research projects of gene therapy, and obtained a number of important research results. The papers were published in top international journals such as Nature, Science and Cell.
China's gene therapy research and clinical trials started almost simultaneously with the developed countries in the world, mainly focusing on major diseases such as cancer and cardiovascular diseases. China has two gene therapy products on the market, mainly for the treatment of malignant tumors of the head and neck. In addition, there are nearly 20 gene therapy products for malignant tumors, cardiovascular diseases and hereditary diseases in China, and 70 clinical trials of gene therapy registered on the Clinical Trial website, accounting for the clinical gene therapy in Asia. 46.7% of the total number of trials (Figure 4). For example, ADV-TK, a tumor gene therapy product developed by Huazhong University of Science and Technology, has significant effects on liver cancer and refractory recurrent head and neck cancer. A multicenter phase III clinical trial is underway. The recombinant human endostatin adenovirus injection (E-10A) developed by Sun Yat-sen University has a good effect in the treatment of advanced head and neck squamous cell carcinoma. Currently, the product has been clinically studied in China and North America, with good development prospects. Ad-HGF injection, a gene therapy product for the treatment of myocardial infarction, developed by the Academy of Military Medical Sciences, has entered Phase II clinical trials, and has been developed in collaboration with Renfu Pharmaceutical Co., Ltd. to obtain a gene therapy product recombinant plasmid-hepatocyte growth factor injection for the treatment of acral ischemia. Phase III clinical approval. Phase II clinical trials of the engineered oncolytic adenovirus gene therapy preparation KH901 for the treatment of head and neck tumors developed by Chengdu Kanghong Biotechnology Co., Ltd. Phase II clinical trials are being conducted in EDS01, a gene therapy product for anti-tumor angiogenesis developed by Sichuan University.
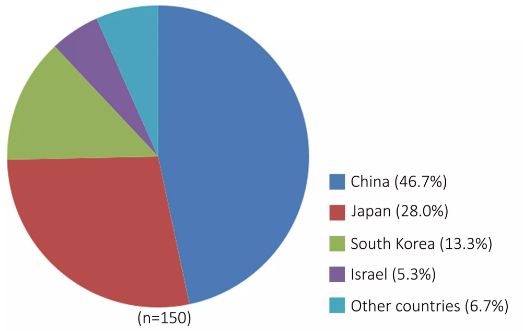
â–² Figure 4: Distribution statistics of clinical trials of gene therapy in major Asian countries
In addition, there are more than 40 gene therapy preparations for major diseases in China in the pre-clinical research stage, and hundreds of projects are in the laboratory research stage.
China is also in the forefront of the world in the field of gene editing and treatment. In April 2015, Huang Jun of Sun Yat-sen University conducted the first genetic editing operation based on CRISPR technology in human embryonic cells, and in September 2017 again reported the use of a single-base editing system to accurately repair specific types in the human embryonic genome. Single base mutation (thalassemia HBB-28). In 2016, West China Hospital of Sichuan University took the lead in the international research on the clinical application of CRISPR/Cas9 gene editing technology in the treatment of lung cancer.
Although the research on gene therapy has made significant progress, there are still some safety and ethical issues that deserve further study. Clinical trials should be strictly in accordance with the approved technical procedures and standards, pay attention to the safety of gene therapy technology, fully protect patients' right to know and protect patient privacy. Although there are still many technical problems to be solved and some legal or ethical issues in gene therapy research, this does not prevent people from having high hopes for curing major diseases.
Source: New Leaf Society
Frozen Swimming Crab,Crab Frozen,Frozen Cut Swimming Crab,Blue Swimming Crab
Zhoushan City Shuangying Aquatic Products Co., Ltd.  , https://www.shuangying-aquatic.com