Release date: 2017-12-01
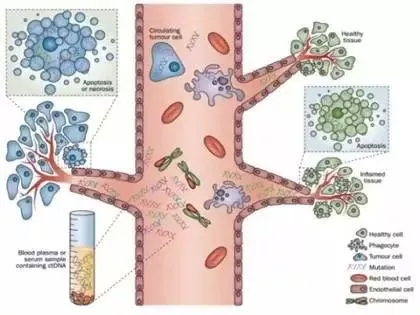
What is a liquid biopsy?
Tumor patients have a small amount of circulating tumor cells in the blood and a small amount of circulating tumor DNA released by necrotic cancer cells. A method of diagnosing and monitoring a patient's tumor by detecting circulating tumor cells (CTC) and circulating tumor DNA (ctDNA) is called a liquid biopsy. In clinical practice, only the surgical biopsy and needle biopsy are available for the tumor samples obtained from tumor patients. Compared with traditional biopsy methods, liquid biopsy has a small side effect, simple operation, and repeated sampling.
The development of liquid biopsy
Liquid biopsies contain CTC and ctDNA techniques.
1. Development history and current status of CTC technology
In 1896, Australian scholar Ashworth first observed tumor cells that had detached from solid tumors and entered the blood circulation in the blood of a patient with metastatic tumors, and first proposed the concept of CTCs. However, the detection of CTCs has not played a role in the prevention and treatment of cancer patients for a long time. The main reason is that the detection technology has not made breakthrough progress. Since the end of the last century, CTCs detection technology has been continuously improved, which is followed by the clinical application of CTCs detection.
1) The first generation of CTC technology: the development history of CellSearch
The first generation of CTC technology used magnetic bead capture. Magnetic beads capable of capturing CTCs were invented by Immunicon in 1983. Immunicon continued to refine the technology and developed specific CTC dyeing techniques. After the company completed a series of clinical trials in 1993-2003, its CTC detection system, Cellsearch, was approved by the US FDA in 2004 for clinical detection of metastatic colorectal cancer, breast cancer and prostate cancer. Veridex, a subsidiary of Johnson & Johnson, acquired Immunicon's CTC business in 2008 and has since grown to this day. The CellSearch system is the only clinical CTC detection system approved by the US FDA. The system was registered with China's CFDA imported device in 2012, making it the only CTC detection system for clinical use in China. Due to its unique clinical use, the Cellsearch system is currently the gold standard for CTC testing.

In clinical testing, the standard procedure is to take 7.5 mL of blood from the patient and check the amount of CTC contained in the Cellsearch system. Clinical data show that patients with normal and benign diseases have very few CTCs, while patients with metastatic disease have different amounts of CTC in the blood according to their severity. By detecting the number of CTCs, the hospital can help the hospital determine the severity of the patient's condition and develop a suitable treatment plan for this.
2) Second generation: the frontier of CTC detection technology
In order to improve the sensitivity of CTC detection and to develop the ability to perform subsequent analysis of CTC cells, a number of technical routes have been adopted for the second generation of CTC testing. Unlike the first generation technology, the second generation CTC is at an early stage both in terms of technology and market. There is no uniform standard in technology, and many companies use different technical routes to capture CTC. It is still difficult to determine which technical route will become the industry standard.
2. Development history and current status of ctDNA technology
In 1948, ctDNA was first found in human blood. The ctDNA of cancer patients was discovered in 1977. In 1994, tumor cells were found to have similar ctDNA to tumor cell genes in vivo. Until 2000, the development of molecular biology and gene sequencing technology has made the mutation detection technology of ctDNA mature, and more and more related research.
From the current research perspective, ctDNA has a wide range of clinical applications, mainly involving early tumor screening, tumor dynamic monitoring, drug resistance mutation detection, evaluation of tumor heterogeneity and risk of recurrence. From the existing clinical experiments, the detection platform of ctDNA is generally the second generation gene sequencing and digital PCR, and the indications are concentrated in common tumors such as non-small cell lung cancer, breast cancer, colorectal cancer and skin cancer. At present, in the domestic market, ctDNA has begun to be used in clinical practice, and Sojin gene has a great advantage in the sensitivity of ctDNA detection technology.
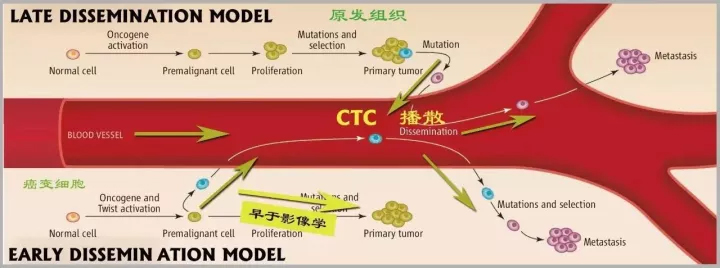
Clinical significance
1, liquid biopsy can solve the difficulties of clinical sampling
Clinically, the patient's tumor tissue samples are obtained by surgical biopsy and biopsy. There may be multiple tumor lesions in patients with metastatic tumors, and it is a big problem to obtain tumor tissue samples from which lesions.
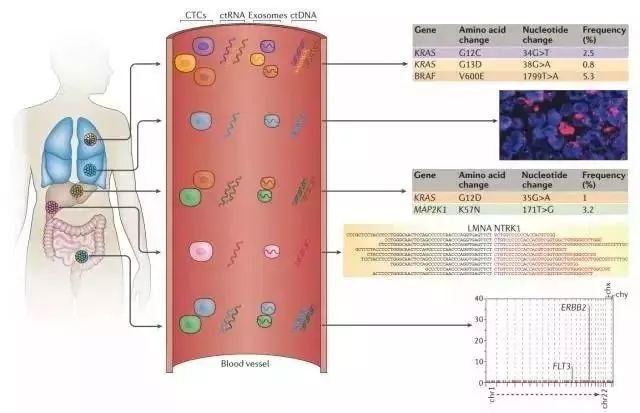
Clinical studies have shown that tumor cells in patients have strong heterogeneity, that is, there are many tumor cells in tumor patients. Different tumor cells have different genotypes, and different treatment options are often needed. In clinical diagnosis, obtaining comprehensive information on tumor cells in patients is the basis of precision medicine. It is assumed that there are 3 tumor lesions in patients with renal cancer, and the tumor cell genotypes are different between different sites. Regardless of which lesion's tumor tissue is obtained by a biopsy, the information obtained is one-sided. However, tumor cells or tumor DNA of each lesion may enter the blood circulation. Collecting CTC or ctDNA through liquid biopsy can obtain comprehensive information on tumor gene or protein expression in patients, and can guide personalized medication more accurately.
2, liquid biopsy can achieve early diagnosis and high frequency monitoring of patients
Tumor cells self-evolve under the influence of drugs to produce drug resistance. The genetic changes of tumor cells are the root cause of drug resistance. It is necessary to perform high-frequency monitoring of tumor gene changes in patients in order to achieve timely and accurate drug use. Surgery and needle biopsy can only be performed up to 2-3 times a year, especially in critically ill patients who are often unable to perform surgery or puncture. Therefore, existing clinical sampling techniques cannot meet the needs of high frequency detection.
However, CTC and ctDNA can obtain tumor cell and DNA information in patients through simple venous blood sampling, which can effectively meet the needs of high frequency monitoring.
In the standard diagnosis and treatment process, the doctor will diagnose and classify the patient's tumor through needle biopsy before surgery and drug treatment, and rely on CT, MRI and other imaging tests to determine whether the patient responds. However, the biopsy has a large limitation in the frequency of detection, and the imaging test has a strong hysteresis in judging the effectiveness of the drug. Liquid biopsy is expected to change the future diagnosis and treatment process. Through continuous high-frequency monitoring, the changes of tumors in patients can be found at any time, and the accuracy of doctors' medications can be improved.
3, liquid biopsy can reduce medical costs
According to US Medicare's analysis of lung cancer biopsy expenses, the cost of ordinary puncture is $8,869. Approximately 20% of biopsy will result in complications, and the cost of needle biopsy and complications will reach $37,745. For medical insurance, the average cost per biopsy is $14,634. However, the first-generation CTC technology Medicare reimbursement is $369, and the second-generation CTC and ctDNA technology spending is about $800-1000. Because it is a blood test, there is generally no complication. From a cost perspective, health insurance has a greater incentive to push CTC and ctDNA technology for liquid biopsy to replace biopsy techniques.
Evaluation
Jose Baselga, chief physician and chief medical officer of the Memorial Sloan Kettering Cancer Center in the United States, said: "This (liquid biopsy) may permanently change the biopsy method, including The response to treatment regimens and the emergence of drug resistance can even be used for early diagnosis in the future.†MIT Technology Review magazine listed “liquid biopsy†as one of the top 10 breakthrough technologies in 2015. The US Clinical Cancer Advance report also believes that liquid biopsy technology is expected to be widely used in the next decade.
Source: Flint Creation
Infusion set,Disposable Syringe,Insulin Syringe,gauze roll,Cotton roll,Sterile Urine cup
FOSHAN PHARMA CO., LTD. , https://www.forepharm.com