A novel HILIC LC-MS method for the detection and quantification of polar cell metabolites
Henry Shion 1 , John Shockcor 1 , Evan Bernier 2 , Stephen Mcdonald 2 , Kirsten Hartil 3 , Bhavapriya Vaitheesvaran 3 , Haitao Lu 3 , Gustavo Palacios 3 , Inwin J. Kurland 3 , Alan L. Millar 1
1 Waters Corporation, Milford, MA; 2 Waters Corporation, Beverly, Massachusetts, USA; 3 Jewish University, Albert Einstein College of Medicine, Bronx, New York, USA
introduction
Complex biological samples typically contain large amounts of endogenous metabolites that reflect the metabolic state of the organism. In particular, skeletal muscle can exhibit a characteristic metabolome "fingerprint" depending on whether it is a fast-twitching muscle (white muscle, glycolysis) or a slow-contracting muscle (red muscle, oxidized). For example, red muscles should show a metabolite fingerprint that is rich in mitochondrial metabolites (such as the Krebs cycle); white muscles should show a metabolite fingerprint that is rich in glycolytic metabolites, and the concentration of mitochondrial metabolites. Very much lower than red muscles. Although many of these metabolites can be highly polar, these samples are typically analyzed using reversed phase liquid chromatography-mass spectrometry (RP-LC-MS), which results in poor retention. In this study, we developed a novel LC-MS method for the detection and quantification of strong polar cell metabolites. In this study, a hydrophilic liquid chromatography (HILIC) based on a sub-2 micron particle BEH amide column was used, and a hybrid quadrupole time-of-flight mass spectrometer and triple quadrupole mass spectrometer were used in parallel.
method
These experiments were performed using a UPLC system using Waters Synapt TM HDMS TM , Waters Synapt G2 HDMS and Xevo TQ:
Liquid chromatography conditions (1 ): using an alkaline mobile phase
LC System: Waters ACQUITY UPLC ® System
- Column: ACQUITY UPLC BEH Amide Amide column, 2.1 x 100 mm, 1.7 μm
- Column temperature: 45 ° C
- Flow rate: 500μL/min
- Alkaline mobile phase A: acetonitrile / water, 95/5 (v / v), 10 mM ammonium acetate, pH = 9.0
- Alkaline mobile phase B: acetonitrile/water, 50/50 (v/v), 10 mM ammonium acetate, pH=9.0
- Gradient (when separated with alkaline mobile phase): Linear gradient, first eluted with 2% B - 85% B for 8 minutes, then held at 98% B for 2 minutes, then returned to 2% B level Rebalance (5 minutes)
- Injection volume: 5-20μL
Liquid chromatography conditions (2 ): use of acidic mobile phase
HPLC System: Waters ® ACQUITY UPLC ® System
- Column: ACQUITY UPLC BEH Amide Amide column, 2.1 x 100 mm, 1.7 μm
- Column temperature: 40 ° C
- Flow rate: 600 μL/min
- Acidic mobile phase A: acetonitrile/water, 95/5 (v/v), 10 mM ammonium acetate, pH=3.0
- Acidic mobile phase B: water, 10 mM ammonium acetate, pH = 3.0
- Gradient: first elute with 5% B - 30% B for 5 minutes, rise to 60% B at 5.5min and hold for 7 minutes, then return to 5% B for rebalancing (5 minutes)
- Injection volume: 20μL full loop
Mass Spectrometry
Synapt HDMS and Synapt G2 HDMS
The mass spectrometer operates in positive ion MS E mode. The capillary voltage used was 3.0 kV, and the source temperature and desolvation temperature were set to 120 ° C and 400 ° C, respectively.
In the MS E acquisition mode, the instrument alternates between alternating low and high collision energy states. This allows simultaneous collection of parent and fragment ion information for all analytes in a single analysis, eliminating sampling biases caused by other conventional methods such as DDA; in conventional methods, the specific m / z must be before fragmentation Was separated.
Xevo-TQ mass spectrometry
The Xevo TQ can operate in both positive and negative ion modes. The capillary voltage was 3.0 kV, and the source temperature and the desolvation temperature were set to 150 ° C and 650 ° C, respectively. The desolvation gas flow rate was set to 1200 L/hr, and the collision gas (argon) flow rate was 0.18 mL/min (4 x 10 -3 mbar). The MS1/MS2 resolution is set to unit mass (0.75 Da FWHM).
Sample preparation of tissue extract
The red soleus muscle (S) was used to compare with the white gastrocnemius (G), which was cut from fasting overnight BALB/c mice and rapidly frozen. The metabolite was extracted by a method of homogenization of the tissue in a 1:1 methanol:water mixed solvent, and the lipid was removed by adding chloroform to the methanol/water extract in a ratio of 1:1. The tissue extract samples after delipidation were separately reconstituted with 400 μL and 4000 μL of a 90:10 acetonitrile:water mixed solvent. Since the content of some components was higher than the upper limit of quantitation, each sample was further diluted by 10 times for analysis.
Results and discussion
1. HILIC LC-MS method development by UPLC using Synapt HDMS
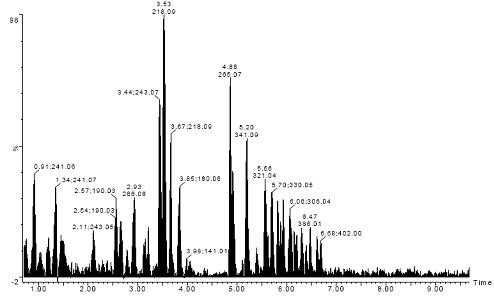
Figure 1. Total ion chromatogram of 108 standard strong polar cell metabolites, including amino acids, DNA/RNA bases and their phosphates, coenzyme A, B vitamins, organic acids, etc.
Figure 1 shows the total ion chromatogram (TIC) of 108 standard strong polar cell metabolites, including amino acids, DNA/RNA bases and their phosphates, coenzyme A, B vitamins, organic acids, and the like. This test was carried out by using Waters UPLC [liquid chromatography conditions (1)] in Waters Synapt HDMS (positive ion MS E mode).
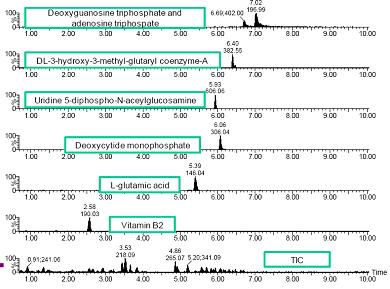
Figure 2. Example of single compound extraction ion chromatogram (within 50mDa window)
Figure 2 shows some examples of extracting ion chromatogram segments (50 mDa window) for a single compound. Most of the peak width measurements in the extracted ion chromatograms selected ranged between 5-15 seconds. However, a tailing peak was also observed with a peak width of up to 60 seconds. Therefore, we consider modifying the conditions of liquid chromatography.
2. Quantitative analysis of HILIC LC-MS method development standards and cell extracts using UPLC / Xevo-TQ instrumentation
Standard analysis performed during UPLC optimization showed that mobile phase buffers with acidic pH increased resolution, signal-to-noise ratio, and peak symmetry for amino acids and organic acids. Figure 3 compares two MRM chromatograms obtained for a pure amino acid solution containing about 2 μg/mL L-lysine. The upper chromatogram is obtained by separation using an alkaline mobile phase (liquid chromatography condition 1), while the lower chromatogram uses an acidic mobile phase (liquid chromatography condition 2).
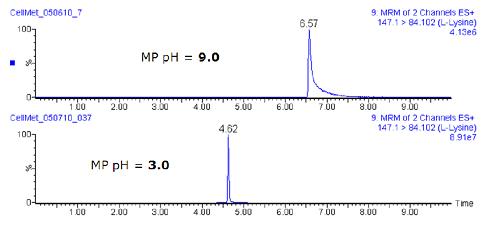
Figure 3. Comparison of two MRM chromatograms containing approximately 2 μg/mL L-lysine solution at different pHs
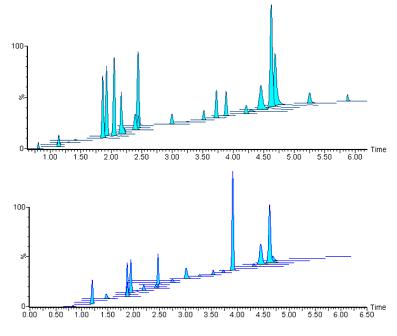
Figure 4. Example of MRM chromatogram obtained from liquid chromatography conditions (2)
Figure 4 overlays the MRM chromatogram of the 1600 ng/mL calibration standard (top panel) and sample HS (0090) in the acidic mobile phase (liquid chromatography conditions (2)).
Under these conditions, we successfully isolated and quantified 28 compounds, including amino acids, organic acids and phosphates (Figure 6).

Figure 5. Example of ADP electronic report from TargetLynx
43 kinds of quantitative analysis of the compound (also including both acidic alkaline conditions) for using TargetLynx TM. For each compound, a corresponding electronic report was generated; the results were determined by linear regression. Figure 5 is a report example of adenosine diphosphate (ADP, which is particularly important in the tricarboxylic acid cycle).
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 6. Summary of calculated concentrations of compounds detected in HS and HG tissue samples using alkaline (left) and acidic (right) mobile phases
Analyte concentrations were calculated for each of the HS and HG tissue samples detected in alkaline and acidic mobile phase separations. A significant difference in relative concentration was confirmed by comparing the metabolite concentrations in the red soleus (S) and gastrocnemius (G) tissue samples.
3. Quantitative analysis of standards and cell extracts of the HILIC LC-MS method by UPLC using Synapt G2
The recently developed QuanTof technology for Synapt G2 [1] and the application of MS E data acquisition technology enable the development of a general-purpose UPLC-MS method that provides quantitative and qualitative analysis of all substances in a single run analysis. Information, including both parent ion information and product ion information. For this determination, serial dilution standards as well as data for HS and HG samples were collected (Figure 7) and all data processing was performed after the acquisition was completed.
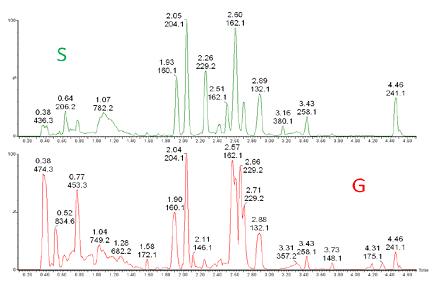
Figure 7. Comparison of full scan chromatograms. Data for S and G cell extracts obtained by UPLC-Synapt G2.

Figure 8. Identification and quantification of target compounds
First, the target compound is automatically screened for the presence of the target compound. Ion identification and validation was achieved by a single processing step using the Posit±veTM software package to derive the exact mass of the parent and daughter ions (2 mDa window) (Figure 8).
This data indicates good agreement with the Xevo TQ analysis results. Figure 8 shows an example in which the compound L-valine is clearly identified in the cell extract and exhibits a linear dynamic range response in 4 orders of magnitude with an RMS mass error of 0.57 mDa.
in conclusion
A novel HILIC LC-MS method for the separation of polar tissue cell metabolites was developed using the novel BEH Amide amide column with a total run time of less than 15 minutes.
Simultaneous quantification of red and white muscle tissue extracts using Xevo TQ and Synapt G2 HDMS clearly distinguishes significant differences between their major metabolites such as AMP , ADP and ATP , and citrulline.
QuanTof technology enables qualitative and quantitative testing to be performed simultaneously on the Synapt G2 .
references
1. Wildgoose J. Integrates an efficient, geometrically folded OA-Tof mass analyzer based on the novel detection technology of ADC. Announcement submitted by the 18th International Mass Spectrometry Conference (IMSC), Bremen, Germany, 2009.
Bluetooth Blood Pressure Monitor
Bluetooth blood pressure monitor,High Quality Bluetooth blood pressure monitor,Bluetooth blood pressure monitor Details, CN
Shenzhen urion Technology Co., Ltd. , https://www.urionhealth.com